Adaptive evolution in biology and technology: Why are parallels expected?
Editor | On 17, May 2003
By: Peter Kaplan
University of Michigan Museum of Paleontology
Ann Arbor, MI 48109
pefty@aya.yale.edu
Introduction
Since the beginnings of technology, inventors have sought to draw parallels between biological and technological designs. Among the flurry of such studies appearing in the 1960’s was Altshuller’s The Innovation Algorithm (1), in which he assembled myriad observations on biological role, function, design, and evolutionary history. Perhaps ironically, Altshuller’s subsequent derivation of laws of technological evolution (2) was without explicit reference to biological systems. However, some workers (e.g., 3,4,5,6), inspired by accumulating admiration of biological design and its applicability to technical problems, sought to understand biological evolution in terms of these technological-evolutionary laws. More recently, an inversion of this paradigm has prompted Mann (7,8) and others (e.g., 6,9,10,11) to ask what technological evolution might gain by emulating biological evolution. Indeed, the notion that biological evolution is somehow “optimal” or “ideal” has stimulated attempts to impose on technological evolution certain characteristic properties of biological evolution.
This optimaliistic motivation is underlain by a rich (but embarrassingly extravagant) tradition in evolutionary biology of adaptive storytelling and marvelling at the functional optimality of organisms to their lifestyles (12). For both evolutionary biologists and TRIZ evolutionists, this perception of an evolutionary ideal to be found in nature may go too far — and lead astray an otherwise productive pursuit. If biological evolution is subideal, then the quest for its emulation in technological evolution may turn out to be a relatively unproductive one. It is worth considering three questions in this connection:
(1) What makes biological evolution potentially subideal — perhaps even less ideal than present-day technological evolution?
(2) Why should we expect biological and technological evolution to behave and proceed similarly or differently?
(3) Is there anything to be gained by emulation of biological evolution (beyond biological form and function themselves)?
These questions can all be most straightforwardly addressed in a discussion of what evolutionary biologists have termed “constraint.”
Evolutionary Constraint
Biological organisms and their evolution constitute non-ideal systems for both intrinsic and extrinsic reasons, as outlined by Seilacher (13). All these reasons are labeled “constraints” on the organism and its evolution, since all act to bias or limit the realizable forms (and functions) or the directions of evolutionary change. First and most basically, organisms are bounded by constructional constraints. For example, it is physically impossible for an organism to reduce its dimensionality to less than three dimensions or change its phase from solid to plasma — even if such a change would be highly beneficial. Second, organisms are subject to historical constraints. For instance, we and other bilaterally symmetric animals are organized along one primary body axis; the hundreds of millions of years of evolution built around this body axis tends to prevent the novel evolution of a multi-axis organism — even if such a design would be physically possible and highly functionally optimal. Third, organisms are beset by functional demands. For example, an organism must maintain a minimum ratio of effective-surface-area-to-volume in order to maintain essential respiratory function. Even if the reduction of this ratio would be functionally favorable for other reasons (e.g., hydrodynamics), such a reduction will be necessarily restricted by the competing functional demand placed by respiration.
A fourth set of constraints is more complex and represents the effective environment occupied by the organism during its evolution and daily function. What is most noteworthy about these constraints is that they vary with the time and place occupied by the organism — much more so than the other forms of constraint. Indeed, the set of environmental conditions under which function evolves (e.g., temperature, humidity, nutrient levels, competition for food, predation pressure) actually fluctuates on the timescale of adaptive evolution. Since changing working conditions demand different optimal designs, functional optima are constantly shifting on the timescales on which organisms evolve. If biological evolution is always occupied with adapting to spatiotemporally local conditions, then it will fail to make progress toward any globally optimal design (14; see 15 for an extended exposition).
Adding yet another dimension, we can think about the range of different environments and working conditions on earth at any one time for organismal designs to adapt to and function in. Again, since different environments may demand different design optima, we should not expect evolutionary “replacement” of one design with another, but rather a coexistence — at the planetary scale — of numerous designs for achieving the same function (16). This situation contrasts drastically with technological evolution, in which environmental variables are normally controlled to allow the globally optimal technology to function anywhere on earth. (Only economic conditions could be said to act in this local way as a control on feasible technological designs.) For these reasons labeled “constraint” and for many other reasons, biological organisms and biological evolution are clearly nonideal. Biological forms and functions are certainly worth emulating in technology, but emulating the typically wandering nature of biological evolution hardly seems worthwhile. We must also recognize that technological evolution should not be expected to behave like biological evolution due to the many differences in the constraint organization of an organism vs. a machine (especially historical and environmental constraint). Despite all these admonitions, it is still worth analyzing the ways in which technological and biological evolutionary patterns do differ. These differences may shed new light on unnecessary nonidealities in the technological evolutionary process, as long as our analysis takes place outside the unproductive realm of emulation of biological evolution.
Figure 1: Evolution of complexity in two biological scenarios (A/D and B/E) and one technological scenario (C/F). Complexity, on the y-axis, might be “number of moving parts”; asterisks represent new record complexity levels through time. Each horizontal bar represents one design; the thin lines connecting them represent genealogical descent, with time proceeding to the right. Note that in (A) and (B) designs are maintained through time, whereas in technological evolution (C), designs replace each other over time.
A: Here complexity increases in 5 of 6 evolutionary branching events, implying a possible trend toward increased complexity.
B: Here complexity increases in only 2 of 6 evolutionary branching events, ruling out a trend toward increased complexity.
C: Here complexity increases, then decreases, as is apparently common in technological evolution.
D,E,F: Plots of maximum complexity through time — akin to Mann’s and my figure 2 — are essentially useless in tracking trends in complexity through time; the three very different scenarios (A,B,C) produce uninformatively similar results here (D,E,F). Even a complexity-increases-then-decreases pattern (like C) would go unrecognized by a maximum-complexity plot (F).
Mann addresses some of these arguments in an intriguing recent paper (8). He contrasts technological and biological evolution and asks why the two seem to differ so strongly. I applaud the clarity of Mann’s framework for comparison, as well as the thorough coverage of known processes in biological evolution. Unfortunately his essay seems to be directed ultimately toward emulation of biological evolution. In addition, the arguments he presents are burdened with several conceptual and factual flaws which threaten to discredit his conclusions. To aid in the pursuit of Mann’s aim — a meaningful comparison between biological and technological evolution — I will deal with these problems one by one. I will conclude with a set of potentially fruitful ways of inquiring about evolutionary patterns and processes.
Assessing Trends in Biological Evolution
Before this specific critique can be launched, I must address a more general point about evolutionary trends and the evidence that can be brought to bear on them. In figure 1a I show an evolutionary tree — a genealogy of biological species. Perhaps the most striking feature of this rather typical biological evolutionary tree is its branching topology. Unlike technological evolution, in which technologies performing the same function typically replace one another over time (Fig. 1c), biological evolution usually proceeds by the advent of new designs followed by long-term coexistence with bearers of past designs. Given this typically non-replacive dynamic, it becomes arbitrary to assess any trend based on only one ancestor-descendant lineage (17). Instead, trends within branching genealogies must be identified by examining changes in multiple lineages (18), not just in the most “progressive” lineage (or, for that matter, not just in the lineage leading to humans). With regard to Mann’s Figure 2 (reprinted here as Figure 2), It is worth noting that most organisms on earth are still bacteria, and that they and the rest of these organisms (and their respective complexities) have not been “replaced” by humans (19). Given the generally non-branching histories of technological evolution (fig. 1c), this discrepancy must be paid special attention in any comparison with biological evolutionary patterns. This is not to say that hints of evolutionary trends are unobservable without a known genealogy. Suppose that we are interested in the evolution of complexity through time in the family of species represented in figure 1a. Even if we do not know the genealogy, we can still use the temporal record (Fig. 1b) to make claims about a general chronology of complexity. For example, the fact that successive increases in complexity occur at later and later times in earth history has been interpreted as evidence for some factor “driving” complexity

Figure 2: Tracking maximum complexity through time. This figure, a reproduction of Mann’s (2003) figure 2, shows the trend in maximum organismal complexity through geologic time. Unfortunately it fails to show a much more salient piece of information: how the distribution of complexity among coexisting organisms behaves from one time to the next through earth history.
upward. However, this vague argument fails on two counts: (i) If complexity starts out low, then the only direction for change — even passive, diffusional change — is toward greater complexity (20). (ii) New record complexity levels must by definition arise in chronological order, so this ordering cannot count as evidence for anything about the history of complexity. My point here is that we must expand our view beyond chronology of new record complexity levels (figure 1d/e/f and figure 2) if we are to move beyond naive arguments about the longterm history of biological complexity. Indeed, figure 1b/e makes this point more forcefully. In this genealogy of species, four out of six complexity changes are reductions, and increases and decreases in complexity level are temporally concurrent; such a history argues against any overarching complexity trend. But even here the new record complexity levels (figure 1e) arise in chronological order! To really drive the point home, even the evolutionary pattern depicted in figure 1c — a clear example of complexity-increases-then-decreases — necessarily shows the same progressive increase in maximum complexity through time (figure 1f). Supposing that an evolving biological system were to manifest a complexity-increases-then-decreases pattern, no graph of maximum complexity through time could be expected to document it. Only by examining multiple ancestor-descendant lineages, or at least by examining temporal changes in the full distribution of complexities, can we hope to say anything empirically meaningful about complexity dynamics in evolving systems.
Mann’s Key Points
Mann (8) begins his essay with a useful discussion of biological evolutionary processes (of which natural selection constitutes only one). With this discussion as backdrop, he makes pictorial reference (his and my figure 2) to an increase in the maximum complexity of life on earth through time. He invokes this progressive increase, with no sign of impending decrease, as a history fit for comparison with that of a technical (i.e., functional) system. The aforementioned “trend” issues notwithstanding, I find it odd to compare the whole branching history of functionally diverse life forms to the technological history of a single functional system. If we aim to ask the same questions about biological evolution as about technological evolution, then we should be examining trends in a single functional structure or mechanism as it is distributed temporally and among species. For example, we might ask whether, over the long-term evolution of predation (21), complexity of jaws and claws has risen or fallen. Alternatively, we might ask about the complexity history of a particular anatomical structure even if its functionality has changed through time (e.g., 22,23). Consideration of the whole organism in such a context might even be justifiable, especially when examining evolution within species or within a lineage of functionally similar species. But asking questions about a whole history of functionally disparate species (bacteria, yeasts, sponges, jellyfish, humans — see figure 2) seems impertinent to any question. Indeed, consideration of the whole history of diverse organismal function would be like asking whether “the technological system” has become more complex. Perhaps some particular systems (e.g., distillation) remain simple after centuries of improvement, while others (e.g., telecommunication) have complexified; but inquiring about the whole lot of “technology” will not address the question at any informative scale. Only by comparing a technology’s complexity to that of its historical predecessor (or genealogical ancestor) can we gain real insight into processes in the evolution of complexity.
As a prime example of what can be gained by examining the evolutionary history of a single functional system, I cite the studies on ammonoid cephalopods executed by Bruce Saunders and colleagues (24, and references therein). This work has focused on a single anatomical structure — the septum (Figure 3) — subjected to consistent functional demands over time, and has shown that the septum’s complexity increased substantially, significantly, continually, and in a “driven” manner over 200,000,000 years. It is worth noting, in this connection, that ammonoids survived for another 180,000,000 years, during which septal complexity is thought to have stopped increasing and manifested no overarching trend for their final 150,000,000 years of evolution.

Figure 3: Ammonoid complexity. The shell of the highly successful but now extinct ammonoids was supported internally by a series of structures known as septa. The septal edges, preserved on many fossils, are known as sutures. Whether topographical complexity is measured as the deviation of the septum from a smooth surface or by the deviation of its suture from a plane curve, ammonoid evolution shows a substantial, significant, continual, and “driven” complexity increase over their 200,000,000-year Paleozoic history (24). (A) The ammonoid animal and shell, with suture lines showing (note: sutures would have functioned in life but would not have shown on exterior of shell). (B) The septum whose edges constitute the suture. (C) Ammonoid sutural complexity through time, with ancestral (300,000,000-year-old) form at top, its 225,000,000-year-old descendant at middle, and its 100,000,000-year-old descendant at bottom. Images modified from Hara-H (41), Oyvind Hammer (42), and Christopher McRoberts (43).
This ammonoid example brings me to another focus of Mann’s essay. Mann’s assertion that “there is no evidence of any natural equivalent of the complexityincreases- then-decreases trend uncovered during TRIZ research” (8: p.2; Mann’s emphasis) is central to his thesis, but have we searched effectively for examples of this trend in nature? Given our tendency to lean on depictions like figure 2 (see critique above) and its new-records-only problem, I wonder if such examples might have been lost in the shuffle. Admittedly, though, I find examples difficult to think of offhand. Probably ammonoid complexity increased-then-decreased — even repeatedly — on certain timescales in certain lineages (25). But since longer-term trends are typically described as such only if they appear monotonic (e.g., 26), it’s likely that biologists have simply ignored any existing u-shaped “trends.” Perhaps a more focused approach will bring to light solid biological examples, or perhaps there are good biological reasons why such a reversal in complexity is unexpected. In either case, the question deserves further exploration and discussion.
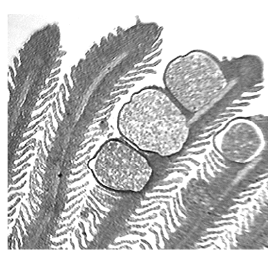
Figure 4: Myxozoa, shown here parasitizing fish gills. Until recently these simple organisms were thought to be mere protozoans, but molecular-evolutionary evidence (32) has now shown that they are actually descended from much more complex cnidarian animals (corals, anemones, etc.). Image courtesy of University of Manitoba Zoology Web (44).
Rare as the increase-then-decrease pattern may or may not be, we must be careful not to confuse it (as Mann does) with the simple evolutionary pattern of complexity reduction. In an attempt to explain the supposed nonexistence of evolutionary reductions in biological complexity, Mann reasons that “there is never a need for a system to become less complex” (p.4). But biological complexity reductions are well-known and relatively common (e.g., 27,28,29)! Many such cases of evolutionary simplification of anatomy (as evidenced by trends in multiple historically independent lineages) are based on complexity measured as “number of parts.” For example, the ancestral tetrapod (an amphibian) had a multitude of skull bones, and subsequent evolution has seen a drastic reduction in the number of bones — in the separate lineages leading toward birds, mammals, and turtles. Other examples range in scale from the classic evolutionary reduction of minimum toe number in horses (30) to the simplified ammonoid septa produced through developmental evolution (31) to the ultrasimplified animal phylum Myxozoa (32,33; see Figure 4). So there is nothing to explain away here; biological evolution commonly produces complexity reductions. Mann’s ready explanation, autopoiesis, is interesting but logically irrelevant, as I shall discuss presently.
Autopoietic Evolution: An Oxymoron
If Mann’s observations are accurate, and the complexity-increases-thendecreases pattern is truly rare in biological evolution, then there are two ways of explaining the discrepancy. First, it might be that technological and biological evolutionary histories are characteristically different. For example, I have noted above that, in biological evolution, functional optima are constantly shifting on evolutionary timescales. Such historical differences between biology and technology might account for observed discrepancies in evolutionary patterns (e.g., complexity-increases-then-decreases).
However, Mann ignores these historical differences and instead searches for differences intrinsic to the evolving entities — i.e., organisms vs. machines. Mann makes the claim that the decisive difference between organisms and machines is autopoiesis — the property of being self-bounded, self-generating, and self-perpetuating. Once Mann defines biological entities as autopoietic, he declares that by definition they must be managing their own levels of complexity, and so there is never a need to reduce complexity (see quote above). I find this argument to be a contortion of just-so storytelling and semantic manipulation. What’s more, Mann fails to consider the essential issue of temporal scale. These argumentational issues only add to the most basic problem with Mann’s reasoning as discussed above: he is attempting to provide an explanation for a nonexistent pattern.
• Just-so storytelling. Mann’s entire argument is set up as an explanation for a supposed trend (monotonic evolutionary increase in organismal complexity) which I have shown above has no theoretical, logical, or evidential basis. The construction of ad hoc explanations is tempting in evolutionary biology but must be avoided, as the explanations themselves act as untestable hypotheses and impede the progress of scientific inference (34).
• Semantic manipulation. Even if there were a trend to be explained, Mann’s means for explanation are axiomatic rather than evidential. He concludes that organisms are managing their own levels of complexity — not through any evidential reasoning about organisms, but merely as a logical consequence of his definition of organisms as autopoietic. Indeed, if organisms do not act autopoietically through evolution (see below), then there is no reason to believe that organisms are autonomously “managing” the evolution of their complexity. Mann also leaves this notion of “management†conveniently undefined.
• Temporal scale. Biological entities may be considered autopoietic on momentary timescales. But the adaptive evolution of organisms can only be effected by forces acting from outside the organism (see Mann’s list of biological-evolutionary processes)! Even random mutation will not direct evolutionary trajectories if there is no natural-selective context in which the mutation can be valued as “good” or “bad” for organismal function. Since this interaction with selective regimes is the sole force behind adaptive (functional) evolution, organisms cannot logically be considered autopoietic on evolutionary timescales. Neither is evolutionary “management” of biocomplexity autopoietic; biological organization, and thus complexity, are necessarily mediated by these same forces acting from outside the organism. Thus, analysis of the biological evolution of function, design, and complexity cannot take place within an autopoietic conceptual framework.
To sum up, evolutionary reductions in biocomplexity are relatively common throughout the history of life on earth — contrary to Mann’s (8) claims. Thus Mann’s portrayal of a monotonic increase in maximum complexity over the history of life is no more than a red herring. Truly driven, monotonic complexity increases are, however, known from single evolving lineages and species; these examples, bounded by historically consistent functional demands, are likely to shed most light on technological evolution. On the other hand, the search for complexity trends over the whole history of life on earth is unlikely to provide any insights for technological evolution beyond the banal “average complexity passively increases over time.” Biological evolution is not “ideal” in its paths’ directness but typically rather wandering. This subideality makes biological evolution a poor model for emulation in technological evolution — especially given the intrinsic differences between the two, in terms of conditions, processes, and evolving entities. Autopoiesis is conceptually inappropriate to analysis of functional evolution, and thus cannot explain the supposed rarity in biological evolution of the complexity-increases-then-decreases pattern. If this pattern is indeed rare, then no valid theoretical arguments have been advanced to explain the rarity. Without a thorough understanding of the data, the theory, and the logical predictions, it is dangerous to go too far with historical or ahistorical explanations in evolutionary biology. We should not be fooled into pursuit of a false idealization of nature out of a poor mischaracterization of nature herself.
Future Directions
Several promising avenues of research into the evolution of biological design and complexity remain open for study. I will outline only a few important directions here; other concepts can be gleaned from Bonner (35), Goodwin (36), and Kauffman (37). First, we can inquire about trends in the functional evolution of particular systems or structures, with or without emphasis on the evolution of complexity (38). In either case, the most convincing evidence will come from analyses within a phylogenetic (genealogical) framework, where multiple, historically independent lineages can be analyzed through time. Second, we can inquire about the total functional variety or versatility of organisms through evolutionary time (39). Third, we can inquire about different ways of measuring complexity (e.g., hierarchicality, meristics [e.g., numbers of cells or cell types] , modularity [number of independently behaving body regions] ) and what our evolutionary expectations should be for each metric (40,20) — for both technological and biological evolution. Fourth, we can inquire as to the biological and technological cost of complexity and how such costs can guide evolutionary trajectories. Assumptions of fixed (or even negative) costs may not hold in all cases, and exceptions may prove most informative. Fifth, Mann’s point concerning autopoietic management of complexity on brief timescales merits futher inquiry — at these timescales. Although this avenue is potentially of less interest to pure technological evolutionists, it is of prime interest to biologists as well as to information and complexity theorists. Finally, we can look for technological examples of branching evolution or of alternative technologies optimal for the same function under different working conditions. These cases may help us better understand the parallels and disparities between technological and biological evolution.
Acknowledgments
I wish to thank Richard Kaplan for alerting me to the potential for mutual illumination between TRIZ and evolutionary biology. I owe my biological inspiration to Carole Hickman, Daniel Fisher, and Geerat Vermeij.
References
1) Altshuller, G.S., 1969: The Innovation Algorithm. Translation published 1999, Worcester: Technical Innovation Center, 205 pp.
2) Altshuller, G.S., 1979: Creativity as an Exact Science. Translation published 1984, New York: Gordon and Breach Science Publishers, 223 pp.
3) Dembski, W.A., 2001: No Free Lunch: Why Specified Complexity Cannot Be Purchased Without Intelligence. Lanham, Maryland: Rowman & Littlefield.
4) Dembski, W.A., a: Becoming a disciplined science: prospects, pitfalls, and a reality check for ID. http://www.arn.org/docs/dembski/wd_disciplinedscience.htm
5) Dembski, W.A., b: ID as a theory of technological evolution. http://www.arn.org/docs/dembski/wd_technoevolution.htm
6) Zlotin, B., Zusman, A., Kaplan, L., Visnepolschi, S., Proseanic, V., and Malkin,
S., 2001: TRIZ Beyond Technology: The theory and practice of applying TRIZ to non-technical areas. TRIZ Journal 2001 (1). https://the-trizjournal.com/archives/2001/01/f/
7) Mann, D., 2001: Integrating knowledge from biology into the TRIZ framework. TRIZ Journal 2001 (10). https://the-trizjournal.com/archives/2001/10/f/
8) Mann, D., 2003: Complexity increases and then… TRIZ Journal 2003 (1). https://the-trizjournal.com/archives/2003/01/a/
9) Michalko, M., 1998: Cracking Creativity. Berkeley: Ten Speed Press.
10) Blosiu, J.O., 2001: Perfection, ideality, and technology road map as measured by a sliding scale (abstract).
http://www.aitriz.org/2001/trizcon2001_abstracts.htm
11) Svetinovic, D., and Godfrey, M., submitted: Attribute-Based Software Evolution: Patterns and Product Line Forecasting.
http://plg.uwaterloo.ca/~migod/papers/attrEvol.pdf
12) Gould, S.J., and Lewontin, R.C., 1979: The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proceedings of the Royal Society of London, B 205: 581-598.
13) Seilacher, A., 1970: Arbeitsakonzept zur Konstruktions-Morphologie. Lethaia 3(4): 393-396.
14) Fisher, D.C., 1986: Progress in organismal design. Life Sciences Research Reports 36: 99-117.
15) Dawkins, R., 1997: Climbing Mount Improbable. New York: Penguin Books, 308 pp.
16) Vermeij, G.J., 1978: Biogeography and Adaptation: Patterns of Marine Life. Cambridge: Harvard University Press.
17) Gould, S.J., 1992: Bully for Brontosaurus. New York: W.W. Norton & Company.
18) Harvey, P.H., and Pagel, M.D., 1991: The Comparative Method in Evolutionary Biology. Oxford: Oxford University Press.
19) Gould, S.J., 1996: Full House: The Spread of Excellence from Plato to Darwin. New York: Harmony Books, 244 pp.
20) McShea, D.W., 1996: Metazoan complexity and evolution: is there a trend? Evolution 50: 477-492.
21) Vermeij, G.J., 1987: Evolution and Escalation: an Ecological History of Life. Princeton: Princeton University Press, 544 pp.
22) McShea, D.W., 1992: A metric for the study of evolutionary trends in the complexity of serial structures. Biological Journal of the Linnean Society 45: 39-55.
23) Fusco, G., and Minelli, A., 2000: Measuring morphological complexity of segmented animals: centipedes as model systems. Journal of Evolutionary Biology 13: 38-46.
24) Saunders, W.B., Work, D.M., and Nikolaeva, S.V., 1999: Evolution of complexity in Paleozoic ammonoid sutures. Science 286: 760-763.
25) Bayer, U., and McGhee, G.R., Jr., 1984: Iterative evolution of Middle Jurassic ammonite faunas. Lethaia 17 (1): 1-16.
26) McNamara, K.J. (ed.), 1990: Evolutionary Trends. Tucson: University of Arizona Press, 368 pp.
27) Leake, J.R., 1994: The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytologist 127 (2): 171-216.
28) Roth, G., Nishikawa, K.C., and Wake, D.B., 1997: Genome size, secondary simplification, and the evolution of the brain in salamanders. Brain, Behavior & Evolution 50 (1): 50-59.
29) Klymkowsky, M., 1999: Relationship between development of human embryo to evolution.
http://www.madsci.org/posts/archives/oct99/940255073.Ev.r.html
30) Matthew, W.D., 1926: The evolution of the horse: a record and its interpretation. The Quarterly Review of Biology 1: 138-180.
31) Wright, C.W., and Kennedy, W.J., 1979: Origin and evolution of the Cretaceous micromorph ammonite family Flickiidae. Palaeontology 22 (3): 685-704.
32) Siddall, M. E., Martin, D.S., Bridge, D., Cone, D.M., and Desser, S.S., 1995: The demise of a phylum of protists: Myxozoa and other parasitic Cnidaria. Journal of Parasitology 81: 961-967.
33) Aleshin, V.V., and Petrov, N.B., 2002: Molecular evidence of regress in evolution of Metazoa. Zhurnal Obshchei Biologii 63 (3): 195-208.
34) Fisher, D.C., 1985: Evolutionary morphology: Beyond the analogous, the anecdotal, and the ad hoc. Paleobiology 11 (1): 120-138
35) Bonner, J.T., 1988: The evolution of complexity by means of natural selection. Princeton: Princeton University Press, 260 pp. 36) Goodwin, B.C., 1994: How the leopard changed its spots : the evolution of
complexity. New York : C. Scribner’s Sons, 252 pp.
37) Kauffman, S., 1996: At Home in the Universe: The Search for Laws of Self- Organization and Complexity. Oxford: Oxford University Press, 321 pp.
38) Hickman, C.S., 1993: Theoretical design space: a new program for the analysis of structural diversity. Pp. 169-182 in Seilacher, A., and Chinzei, K., eds.: Progress in Constructional Morphology. Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 190 (2-3).
39) Vermeij, G.J., 1973: Biological versatility and earth history. Proceedings of the National Academy of Sciiences of the United States of America 70: 1936-1938.
40) Atlan, H., 1988: Measures of biologically meaningful complexity. Pp. 112-127 in Peliti, L., and Vulpiani, A.: Measures of Complexity. Lecture Notes in Physics 314.
41) Hara-H, 2000: untitled. http://www.asahi-net.or.jp/~zq2h-hr/Denga/Mange1/Ammonite.JPG
42) Hammer, O., a: Modelling ammonoid septae. http://www.notam02.no/~oyvindha/septum.html
43) McRoberts, C., 1998: Cephalopods. http://paleo.cortland.edu/tutorial/ Ceph&Gast/cephalopods.htm
44) University of Manitoba Department of Zoology, 2000: Myxozoa spp. http:// www.umanitoba.ca/faculties/science/zoology/faculty/dick/z346/myxohome. html


