40 Principles – Chemical Illustrations
Editor | On 02, Jul 2003
By: Billy Grierson, Iain Fraser, Ailsa Morrison, Stuart Niven, Greig Chisholm
Ciba Specialty Chemicals
Contact: billy.grierson@cibasc.com
Introduction
The origins of TRIZ are to be found in the analysis of engineering and utility patents of the former USSR. As such the 40 Principles of TRIZ were constructed to tackle engineering problems. Much work has been done attempting to extend the use of the principles into non-engineering situations, such as the ‘soft’ issues in business, personal or health. However, there has been less work published which aims TRIZ directly at chemistry and its applications. This paper is designed to initiate the discussion on how the 40 principles of TRIZ may be applied directly to chemical problems.
Background
In Ciba we have tried to apply TRIZ to chemical problems but have found it difficult to relate the 40 Principles to what happens in a chemical system. The main challenge we have experienced in applying TRIZ to chemistry is finding examples that can be used to explain the meaning of the 40 Principles in chemical terms. Most of the examples currently used are engineering systems that do not always have an obvious relevance to the molecular world.
In order to try to overcome this hurdle we have put together some illustrations based on chemistry and its applications rather than engineering. We have tried to use general chemical reactions or principles as far as possible, but coming from a pigment manufacturing background there are inevitably some examples which are specific to the colour industry. Full explanations of all examples given can be found in generally available chemical textbooks (see below) and most should be familiar to any chemist with a good general knowledge of the subject.
This list is not intended as an aid to the extension of TRIZ into chemistry, but rather as a teaching tool to help introduce TRIZ to chemists in terms they understand. We would be greatly interested in any other suggestions for illustrations, or any case studies involving chemical systems.
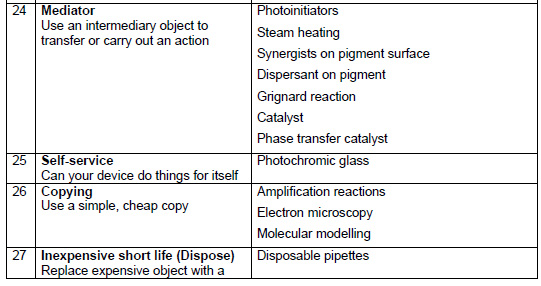
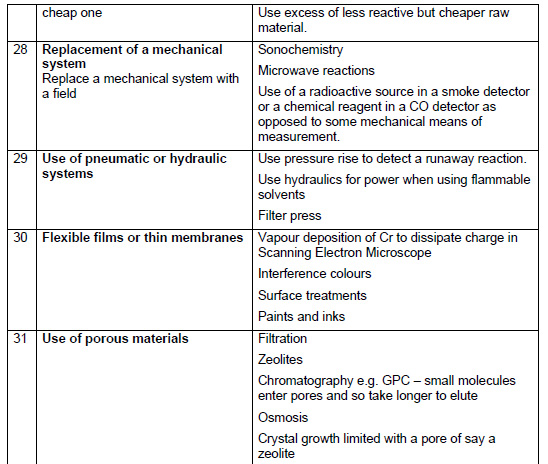
Chemical Illustrations of the 40 Principles of TRIZ
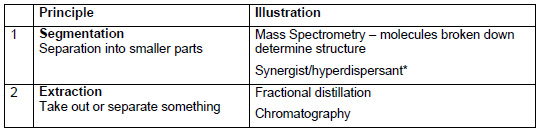
* The term synergist/hyperdispersant is familiar to anyone working in the ink or surface coating industry. In principle, a dispersion of pigment particles in a liquid medium will tend to flocculate unless there is some stabilisation mechanism. In non-aqueous systems, this is achieved by adsorbing a solvent soluble chain onto the pigment surface. This creates a barrier which prevents particles from approaching close enough to interact. However, if the adsorbing chain is soluble, it will stay in solution and not adsorb onto the pigment. What is needed is an insoluble soluble surface treatment. The principle of segmentation is used by having a two part system. One part, the “synergistâ€, is insoluble and strongly adsorbs onto the pigment. This synergist contains polar groups. The dispersant consists of a soluble chain, with a polar end group. Overall, the dispersant is soluble in the medium, but the polar end group interacts strongly with the synergist and so is held close to the surface of the pigment. The soluble chain of the dispersant sticks out into the medium and gives a barrier to flocculation.
An alternative approach is to use ABA block co-polymers. The A blocks consist of solvent soluble chains, whereas the B block is insoluble. The B block will sit on the pigment surface and hold the A blocks in position to create the barrier.
Bibliography
Kirk Othmer Encyclopaedia of Chemical Technology, 3rd Edition. 1982. Wiley Interscience.
The Printing Ink Manual, edited by R.H. Leach & R.J. Peirce, 5th Edition. 1993. Blueprint.
Color Chemistry, H. Zollinger. 2nd, Revised. 1991. VCH.
Perry’s Chemical Engineers’ Handbook, Robert H. Perry, Don Green, 6th Edition. 1984.
McGraw Hill.
Chemistry – The Central Science, 9th Edition, Brown, LeMay, Bursten & Burdge. 2003.
Prentice-Hall.